About the EMPOWER Studies
The EMPOWER program includes three clinical research studies to test the effectiveness, safety and tolerability of an investigational drug called emraclidine.
EMPOWER-1 (CVL-231-2001) & EMPOWER-2 (CVL-231-2002)
EMPOWER-1 and EMPOWER-2 are evaluating emraclidine in people living with schizophrenia who are experiencing an acute episode of psychosis.
In this study, 2 out of every 3 participants will receive the investigational drug while 1 of 3 will receive placebo. Neither the participants, nor the study doctors, will know who is getting which treatment.
Both studies are in-patient trials with a 2-3 week screening period followed by a 6-week treatment period. In addition, participants in this study may be eligible to enroll in EMPOWER-3, a long-term study in which all participants will receive the study drug for 52 weeks.
EMPOWER-3 (CVL-231-2003)
EMPOWER-3 is evaluating emraclidine in people living with schizophrenia who have stable symptoms and are not currently experiencing an acute episode of psychosis. The study is enrolling people from two different groups:
- People living with schizophrenia who rolled over from EMPOWER-1 or EMPOWER-2
- People living with schizophrenia who did not enroll in EMPOWER-1 or EMPOWER-2 and meet the eligibility requirements
In this study, all participants will receive the investigational drug for a 52-week treatment period. There is no placebo in this study.
About Schizophrenia
Schizophrenia is a serious mental illness characterized by positive, negative and cognitive symptoms, including delusions, hallucinations, disorganized speech or behavior, slowed speech and blunted affect.i, ii, iii, iv
The symptoms of schizophrenia are thought to result from the brain’s inability to regulate the neurotransmitter dopamine. Currently available therapies for schizophrenia work primarily by blocking dopamine receptors, which sometimes results in challenging side effects. These side effects are associated with people stopping their medications which can lead to high relapse rates, worsening symptoms and progression of disease.
i Tandon R, Gaebel W, Barch DM, et al. Definition and description of schizophrenia in the DSM-5. Schizophr Res. 2013;150(1):3-10.
ii Marder SR, Cannon TD. Schizophrenia. N Engl J Med. 2019;381(18):1753-1761.
iii American Psychiatric Association. The American Psychiatric Association Practice Guideline for the Treatment of Patients With Schizophrenia; third edition; 2021.
iv Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261-276
Science of Emraclidine
Emraclidine is an investigational drug being evaluated for the treatment of schizophrenia.
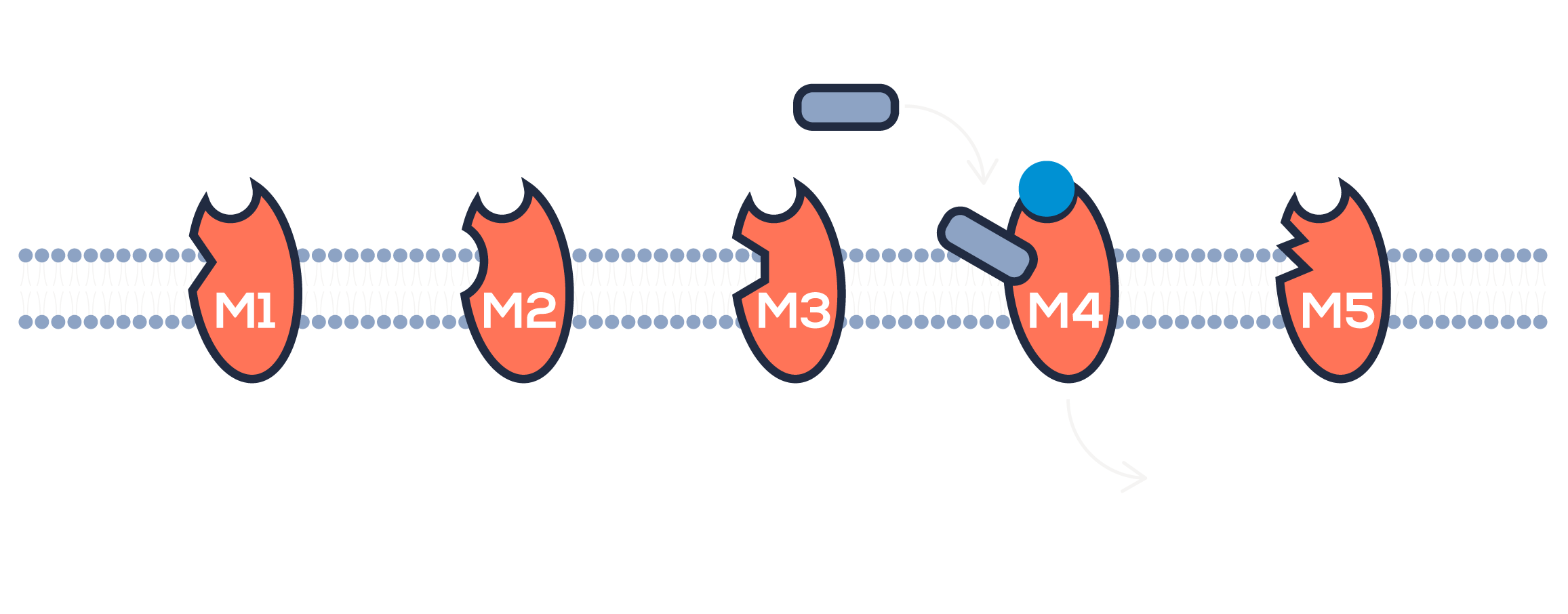
Emraclidine is thought to work differently from current antipsychotics. Instead of blocking dopamine receptors, emraclidine targets a different system entirely called the muscarinic system. Emraclidine is a M4 positive allosteric modulator, meaning that it helps enhance the activity at a specific receptor, called the M4 muscarinic acetylcholine receptor. Activity at this receptor is thought to reduce excess levels of the neurotransmitter dopamine and may potentially reduce symptoms of schizophrenia without causing many of the side effects seen with therapies that directly block dopamine receptors.
Learn More About the EMPOWER Studies
dopamine = a neurotransmitter that activates various aspects of human emotion and behavior. Excess dopamine is associated with symptoms of schizophrenia.
positive allosteric modulator = a molecule that enhances the activity of a receptor, without directly activating it.
muscarinic acetylcholine receptors = receptors that are widely distributed throughout the body and mediate functions of the body according to location and receptor subtype.
M4 receptors = a specific subtype of muscarinic acetylcholine receptors that are located primarily in the brain and believed to play a role in limiting excess dopamine release.
PANSS = Positive and Negative Symptom Scale – clinically validated scale for assessing symptoms of schizophrenia.
CGI-S = Clinical Global Impression Scale–Severity – a clinically validated scale for how a doctor views the severity of an illness.
Who is Eligible?
EMPOWER-1 and EMPOWER-2 Eligibility*
- Adults 18–65 years old
- Diagnosis of schizophrenia by a doctor
- Experiencing an acute exacerbation or relapse of symptoms
- PANSS Total Score between 85 and 120, inclusive
- CGI-S ≥4
*This is not an exhaustive list. A full evaluation will be conducted by the study location.
EMPOWER-3 Eligibility*
- Adults 18–65 years old
- Diagnosis of schizophrenia by a doctor
- Have been on a stable schizophrenia medication for at least one 3-month period in the year prior
- Not currently receiving treatment in a clinic
If you or someone you know may be eligible for one of the EMPOWER studies, please contact a participating site for further evaluation.
Find a Study Location
To begin your search choose from the EMPOWER-1 and EMPOWER-2 or EMPOWER-3 studies, enter your address and/or zip code.
Interested in becoming a study location? Contact us at:
Frequently Asked Questions
Clinical research studies, also known as clinical trials, are scientific investigations that shape the future of medicine by helping to find:
- New medicines
- Better formulations of existing medicines
- New uses for existing medicines
Before introducing new medicine to the public, pharmaceutical companies must test their investigational drugs very carefully through approved medical research studies (also called “clinical trials”). Study doctors are required to follow strict rules to protect the safety of the people who volunteer to participate in research studies. All researchers must follow a detailed plan, called a protocol, which explains all study procedures. Protocols are reviewed by an independent board (or group of people) that oversees the safety of all study participants. Additionally, before enrolling individuals into a study, researchers must fully explain the study to the individual and answer any questions they may have. Participation in a research study is voluntary and individuals may withdraw from the study at any time for any reason. Participants may or may not benefit from the investigational drug.
Global regulatory agencies, such as the United States Food and Drug Administration (FDA), thoroughly review the data collected in these studies before authorizing new medications for the public. Once a drug is authorized, many pharmaceutical companies continue to conduct research on the authorized drug to collect ongoing safety and effectiveness data.
The study doctor and staff will review a potential participant’s medical history to determine eligibility to participate. If eligible, the participant will be asked to read and sign an Informed Consent Form. This form contains information about the study, a summary of what may be expected during the study participation, potential risks and benefits, the rights of study participants and other options that may be available to the participant.
Participation in EMPOWER-1 and EMPOWER-2 lasts approx. 10 weeks and participation in EMPOWER-3 lasts 52 weeks.
A stipend is provided to cover the costs for time spent in the clinic during the treatment period and any travel. Additionally, all examinations, evaluations and treatments associated with the EMPOWER studies are provided at no cost.

